Introduction
Despite multiple awareness-raising initiatives, and in contrast with male persons with hemophilia (PwH), a considerable number of carriers and female PwH still go undiagnosed. We here present the findings of a proactive and extensive hemophilia carrier screening project.
Methods
From May 2021 until April 2023, potential and obligate carriers of hemophilia A (HA) and B (HB) were identified by updating family trees of all PwH regularly followed at the Cliniques universitaires Saint-Luc. Data from previously screened female individuals (bleeding history, coagulation factor assays and testing for the index patient's pathological genetic variant) were collected retrospectively through electronic medical records (EMRs). Female individuals who had not been previously screened or with missing data were provided with a total of 125 invitation letters by related index hemophilia patients. The study protocol received approval of the local ethics committee.
Results
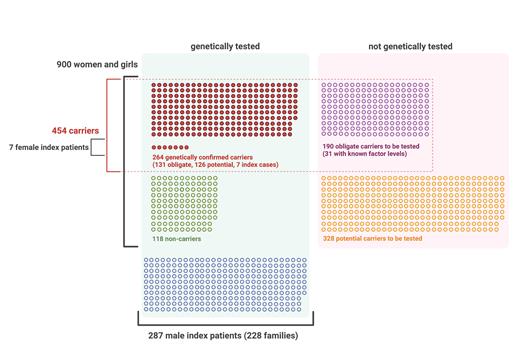
All 287 male PwH (226 HA, 61 HB) from 228 families (180 HA, 48 HB) were included in the analysis. Seven female PwH (3 HA, 4 HB) were considered as index cases in their families. In total, based on the family trees, there were 900 females, among which 454 were obligate and/or genetically proven carriers of hemophilia and 118 were found to be non-carriers (Figure 1). Mean age at diagnosis was 30.7 years (range 1-82) in families with HA and 30.1 years (range 0.5-93) in families with HB, without significant difference between these two groups (p=0.809) or between families with severe, moderate or mild hemophilia (p=0.535).
Genetic testing for pathological variants has been conducted in 133 obligate, 237 potential and 4 sporadic carriers, and remains to be performed in 190 obligate and 328 potential carriers. Eight females were obligate non-carriers. The group of mothers, sisters and daughters of PwH comprised 543 females, of which at least 178 (35.7% of those with known date of birth) were of reproductive age (18-49 years). Seventy-three percent of females in the latter group have had genetic testing. The proportion of female individuals genetically tested did not significantly differ between families of different severities of HA (p=0.132). In families with moderate HB, female individuals were more likely to be tested (genetic status known in 59.4%) than in other HB severity groups (p=0.01), yet this may be attributed to the presence of one large family comprising 6 male persons with moderate HB and 22 female individuals, of whom a large proportion (n=18, 82%) has had genetic testing.
Among carriers with known coagulation factor levels (261/454), 42 (23.0%) and 23 (29.5%) carriers of HA and HB, respectively, had a factor level below 40 IU/dL, and 65 (35.5 %) and 32 (41.0%) had a factor level below 50 IU/dL. The proportion of carriers with a factor level below 40 IU/dL did not significantly differ between families with severe, moderate and mild hemophilia (22.6%, 34.7% and 22.7% respectively, p=0.213), neither did mean coagulation factor levels in carriers with a factor deficiency in these groups (26.2, 30.4 and 27.1 IU/dL in families with severe, moderate and mild HA and HB, p=0.344). Carriers with a coagulation factor deficiency of less than 40 IU/dL were screened earlier than other female individuals (mean age at diagnosis 25.8 and 31.8 years, respectively, p=0.034).
Conclusions
This study represents the first reported attempt to systematically identify all (potential) hemophilia carriers within families of all PwH followed at a single hemophilia treatment center. To date, this initiative has allowed 44% of the entire cohort to be screened, and carrier status to be genetically confirmed in 73% of mothers, sisters and daughters of PwH who are of childbearing age, based on the date of birth documented in their EMR.
Although original and promising, these data reflect the major challenges and difficulties in reaching out and providing appropriate screening to all women genetically linked to one or more PwH. The carrier screening efforts that have been initiated in our center, and which should ideally be replicated across the hemophilia community globally, appear as a critical step towards providing equal access to haemophilia diagnosis and care to all potentially affected individuals, regardless of their gender.
Disclosures
Hermans:Bayer, Takeda, Roche, CSL Behring, Novo Nordisk, Pfizer, Sobi, LFB, OctaPharma, Uniqure and Biomarin: Consultancy.